From snakes to snails and whelks to whales, animals’ body plans are a blueprint for the way they live.
Whether they have legs or wings, claws or antennae, a shell or scales – the body plan is what maps how a creature can move, and frames how it feeds and survives.
A body plan describes how an animal is put together and the way it develops from embryo to adult, providing a road map for tracing the evolutionary routes of different species.
But how and when did such a diversity of body plans come into being? Why does every corner of our planet wriggle with animal life?
This website accompanies the First Animals exhibition at Oxford University Museum of Natural History, which explores the strands of evidence for Earth’s mysterious early animals. Trace the dramatic steps in which all the animal body plans we have today emerged. And see the unique fossil remains that reveal a burst of evolutionary activity called the Cambrian explosion, 540 million years ago.
The earliest animals left no fossil remains. What were they like?
The first animals – including the common ancestor of all animals today – evolved in the sea over half a billion years ago. We have no direct evidence of what they were like.
But by studying animals today, we can work out features they must have shared – small size, soft bodies, and a tendency to stay very still or creep slowly across the ocean floor.
How were these life forms different and new?
The creatures had bodies built from multiple cells with specialised roles, like organisms before them. Now, those cells could also form sheets called epithelia, allowing structures to develop. Along with increased genetic complexity, this set the scene for big changes.

Millimetre-long placozoans were among the earliest animals to emerge, yet they could feed, digest, reproduce, and move around the ocean floor. Their structure is like a sandwich, with two outer sheets of cells and a liquid filling supported by structural fibre cells.
Millimetre-long placozoans were among the earliest animals to emerge, yet they could feed, digest, reproduce, and move around the ocean floor. Their structure is like a sandwich, with two outer sheets of cells and a liquid filling supported by structural fibre cells.

Ediacaran sea floor, Newfoundland, Canada - 560 million years ago. Image: Mighty Fossils
Ediacaran sea floor, Newfoundland, Canada - 560 million years ago. Image: Mighty Fossils
When did Earth turn into an action-packed animal planet?
About 540 million years ago, life became considerably more dynamic. In a burst of activity called the Cambrian explosion, the planet transformed into a slithering, swimming, scuttling place.
Among countless new species were examples of animals with every body plan we recognise today. What triggered such a dazzling development?
Earth’s environment was in flux during the Cambrian period, and the Ediacaran period that came before it. Sea levels rose, and chemicals washed into the ocean. In the underwater world, evolution got to work. New creatures emerged that could move further than ever before – and change their environment by burrowing and building. Soon, the new species were living in every habitat across the length and breadth of the ocean.
How can we trace the earliest animals?
The Ediacaran and Cambrian periods were around half a billion years ago. But hidden in the genetic code of modern animals are signs of when the major animal groups first arose.
Are there any clues to animal behaviour and body layout?
Yes. Trace fossils are marks of physical movement and activity preserved in rocks. Burrows indicate some creatures had a gut that ran all the way through their bodies, like worms do. Tracks offer evidence of animals that are symmetrical on either side of their length. This group of animals, the bilaterians, forms the majority of the animal kingdom today.

Life has also left its mark via chemical traces in
Earth’s rocky layers. Faint biomarkers can include
substances such as cholesterol, essential for
animal cells, like the pancreatic cell above, to
function and a potential indicator of animal life.
© BIOPHOTO ASSOCIATES / SCIENCE PHOTO LIBRARY
Life has also left its mark via chemical traces in
Earth’s rocky layers. Faint biomarkers can include
substances such as cholesterol, essential for
animal cells, like the pancreatic cell above, to
function and a potential indicator of animal life.
© BIOPHOTO ASSOCIATES / SCIENCE PHOTO LIBRARY
Genetic evidence is helping reveal the timescale of animal evolution. We can predict the rates of change to genetic code through time, a little like the ticking of a clock. By counting the number of differences between the same sections of code in different species, you can estimate how long ago they branched away from each other.
The latest research using these molecular clocks places the earliest animals around 100 million years older than fossils imply, before the Ediacaran period. This apparent conflict between rocks versus clocks tells us a lot about this earliest period of animal evolution – the time between the very first animals and those that had evolved the features we recognise as belonging to the animals we know today.
Intriguingly, an early branching point could have been triggered by climate change. Several times just before the Ediacaran, the Snowball Earth phenomenon occurred – runaway global cooling. This may have put organisms under extra pressure to adapt and also resulted in an environment poised and ready for animal diversification.

Ediacaran sea floor, Namibia, Southwest Africa - 543 million years ago. Image: Mighty Fossils
Ediacaran sea floor, Namibia, Southwest Africa - 543 million years ago. Image: Mighty Fossils
When did animals first appear?
An animal, a fungus, an alga… or something else entirely that no longer exists? The unfamiliar species of the Ediacaran period are hard to classify. By reconstructing different aspects of the biology of these strange fossilised animals, we can search for clues to link them to living groups.
So what is the definitive evidence of early animal life?
Researchers seek clues in Ediacaran fossils that could indicate the remains of an early animal. They look for variety in tissue types, possibly with muscle tissue and epithelial cells that form sheets. There could be a gut that runs end-to-end, or evidence of eating other species. Left-right symmetry, the presence of a nervous system, and traces of cholesterol are further signs of animal life.
Animal, vegetable or mineral?
Clues to life in the Ediacaran period, which began 635 million years ago, have turned up in rocks from all around the world, including the Ediacara Hills of South Australia from which the period takes its name. The flattened casts and moulds of these soft-bodied organisms can be hard to reimagine as living things, but scientists interpret all the species displayed in this case as early animals.

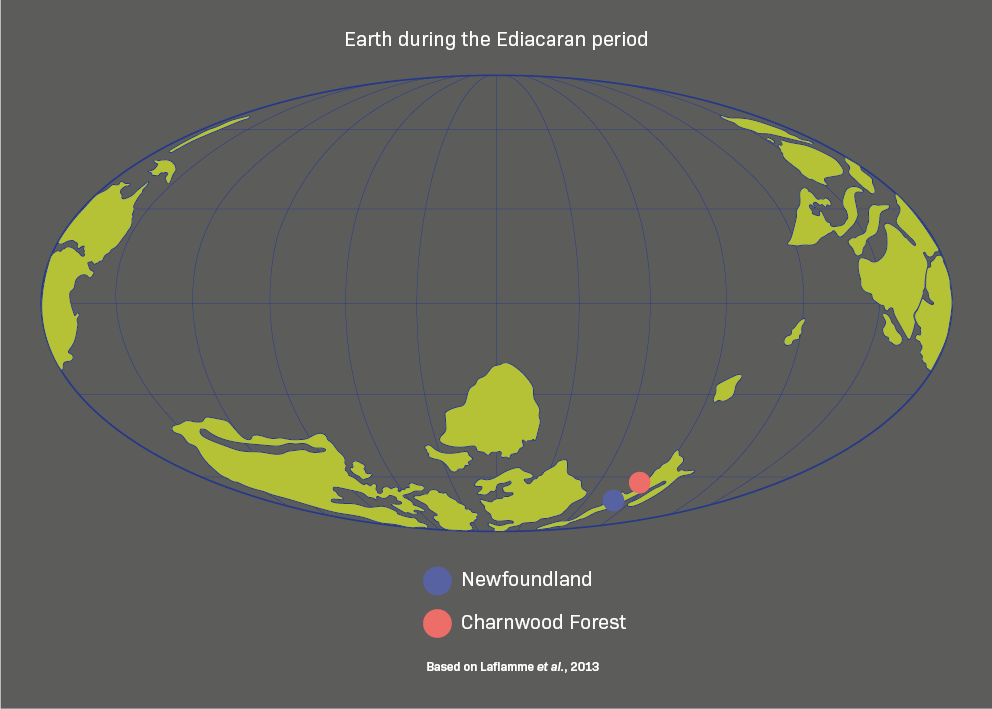
Charnia lived on the sea floor during the Ediacaran period and is now preserved in this spectacular fossil bed in Newfoundland, Canada – a UNESCO World Heritage site.
Charnia lived on the sea floor during the Ediacaran period and is now preserved in this spectacular fossil bed in Newfoundland, Canada – a UNESCO World Heritage site.
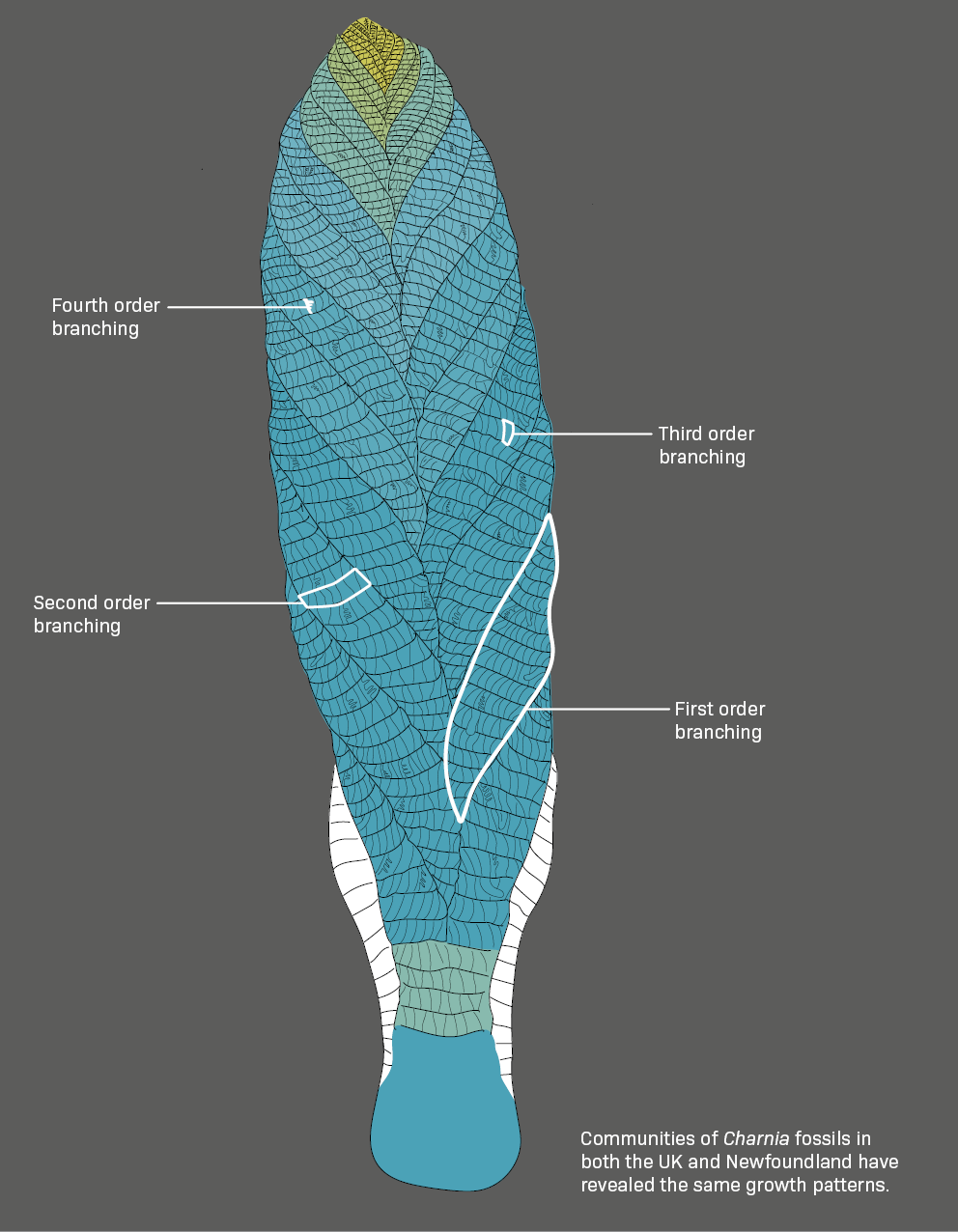
Is Charnia really an animal?
Charnia may look like a plant, but new research is providing definitive evidence that it was an animal. It is a key species of the Ediacaran period – one of the oldest, widest-ranging, and geologically longest-lived – and often appears in varying sizes in the same location.
Comparison is the key to the scientists’ findings, revealing a growth pattern that is animal-like rather than plant-like. Small Charnia grow by adding branches at the tip of the body. But at a certain point, they stop, and instead make the existing branches bigger. This developmental shift reflects the way animals grow from juveniles into adults.
Frankie Dunn, Research Fellow at Oxford University Museum of Natural History, tackles the tricky question 'What is an animal?'
Frankie Dunn, Research Fellow at Oxford University Museum of Natural History, tackles the tricky question 'What is an animal?'
What is special about an exceptionally preserved fossil?
Most animal bodies rot away without trace. On a few occasions, casts are captured when bones or shells fall into sediment before they decay, creating the familiar kind of fossils.
But exceptionally preserved fossils are the rarest of the rare. They feature soft or non-bony tissues that usually disappear quickly after death – muscles, nerves, guts, scales. In certain conditions, hard minerals replace the original proteins or sugars in these delicate tissues, preserving their structure.
The anatomical clues that the soft tissues provide are vital for fitting extinct animals into the evolutionary family tree in the right way.

Misszhouia longicaudata had no hard skeleton, but it does show exceptional preservation of soft tissues. The dark stripe running down the middle of the specimen is its gut, while the fine ridges on the head and body reveal rare evidence of its delicate legs. Conditions that produce incredible fossils like this are very rare but usually include rapid burial of the creature after death, to prevent disturbance by marine currents and scavengers, and limit oxygen to slow down decay.
Misszhouia longicaudata had no hard skeleton, but it does show exceptional preservation of soft tissues. The dark stripe running down the middle of the specimen is its gut, while the fine ridges on the head and body reveal rare evidence of its delicate legs. Conditions that produce incredible fossils like this are very rare but usually include rapid burial of the creature after death, to prevent disturbance by marine currents and scavengers, and limit oxygen to slow down decay.
Dr Imran Rahman, Deputy Head of Research at Oxford University Museum of Natural History, explains how palaeontologists reconstruct extinct animals from fossil evidence, using the example of Vetulicola cuneata from Chengjiang.
Dr Imran Rahman, Deputy Head of Research at Oxford University Museum of Natural History, explains how palaeontologists reconstruct extinct animals from fossil evidence, using the example of Vetulicola cuneata from Chengjiang.
How did animal bodies change as they evolved?
Soft and squishy to active and armoured – the new animal species were a world away from their placid predecessors. A chemical clue to this change was in the water. A major rise in sea level washed calcium, phosphate and other life-essential chemicals into the ocean. Animals were able to build skeletons and shells for the first time.
What was the impact of this chemical boost?
Animals could now move and feed in new ways, grow larger and dig deeper. They could also protect themselves with armour, spicules and spines, and hunt using teeth and claws. An arms race began between predator and prey, accelerating change that opened up even more ecological niches in which to thrive.

With the chemicals in the seawater around them,
molluscs could start to make substances such
as mother-of-pearl, the iridescent coating inside
their shells. In this scanning electron micrograph
you can see the sheets formed from microscopic
hexagonal plates made of calcium carbonate.
© EYE OF SCIENCE / SCIENCE PHOTO LIBRARY
With the chemicals in the seawater around them,
molluscs could start to make substances such
as mother-of-pearl, the iridescent coating inside
their shells. In this scanning electron micrograph
you can see the sheets formed from microscopic
hexagonal plates made of calcium carbonate.
© EYE OF SCIENCE / SCIENCE PHOTO LIBRARY
Animals toughen up
In our crab-eat-crab world, many species still use chemical elements in the water to build hard structures. We don’t know why it began – were animals taking advantage of the new chemicals flooding into the sea? Or were they trying to lock them away in mineralised tissues because they found them toxic?
Shells, spines, plates and tubes
All these elements of modern animal skeletons appear in the microscopic specimens called Small Shelly Fossils. Some are the remains of entire creatures. Others are like jigsaws with more pieces lost than found. But all help to show the form and function of the hard parts that animals evolved during the late Ediacaran and early Cambrian.
To reveal the secrets locked inside these tiny fossils, researchers use an X-ray scanning technique to build three-dimensional models of the specimens. The scientists can then peel the layers away and reconstruct how a skeleton changed as it grew – digitally slicing through at any angle for more information.
Duncan Murdock, research fellow at Oxford University Museum of Natural History, on when animals first evolved skeletons
Duncan Murdock, research fellow at Oxford University Museum of Natural History, on when animals first evolved skeletons
What do the world’s oldest animal skeletons look like?
The earliest fossil specimens come from around 550 million years ago, during the late Ediacaran period. Tiny examples, called microfossils, are too small to see clearly with the naked eye but reveal a huge amount about the early evolution of animals and how different groups relate to one another.
The creatures’ ability to incorporate chemical compounds from the environment into their skeletons led to fundamental changes in their structure and lifestyles.

Namacalathus is one of the oldest-known examples in the fossil record of a creature that built a skeleton. The animal was the shape of a goblet on a stalk and may be part of the larger group that includes molluscs.
Namacalathus is one of the oldest-known examples in the fossil record of a creature that built a skeleton. The animal was the shape of a goblet on a stalk and may be part of the larger group that includes molluscs.

Animals such as Protohertzina developed strong spines for hunting (top image) in response to the hard protective shells of genera such as Pelagiella (bottom image) which also had defensive spines of its own. These fossils are from Iran, and are coated in gold to assist with imaging.
Animals such as Protohertzina developed strong spines for hunting (top image) in response to the hard protective shells of genera such as Pelagiella (bottom image) which also had defensive spines of its own. These fossils are from Iran, and are coated in gold to assist with imaging.
WHAT IS THE BEST EVIDENCE FOR THE CAMBRIAN EXPLOSION?
Brought together here for the first time, from Greenland, China and Canada, is extraordinary fossil evidence of the diversity of the animal life that evolved during the Cambrian explosion. Some types of animals appear uniquely in one location, while others turn up in all three of these far-flung places.
Can we see these animals in the wild now?
No – they are all extinct. But vitally, there are early representatives of every animal body plan that exists today – those with fins, shells and legs, plus the forerunners of everything soaring, scampering, scuttling, slithering and swimming. The fossils here are exceptionally preserved, retaining rare details of the soft tissue as well as hard parts.
Xiaoya Ma, senior research fellow at the University of Exeter, reveals what fossils can tell us about the earliest animals
Xiaoya Ma, senior research fellow at the University of Exeter, reveals what fossils can tell us about the earliest animals

Cambrian sea floor, Yunnan Province, China - 518 million years ago. Image: Mighty Fossils
Cambrian sea floor, Yunnan Province, China - 518 million years ago. Image: Mighty Fossils
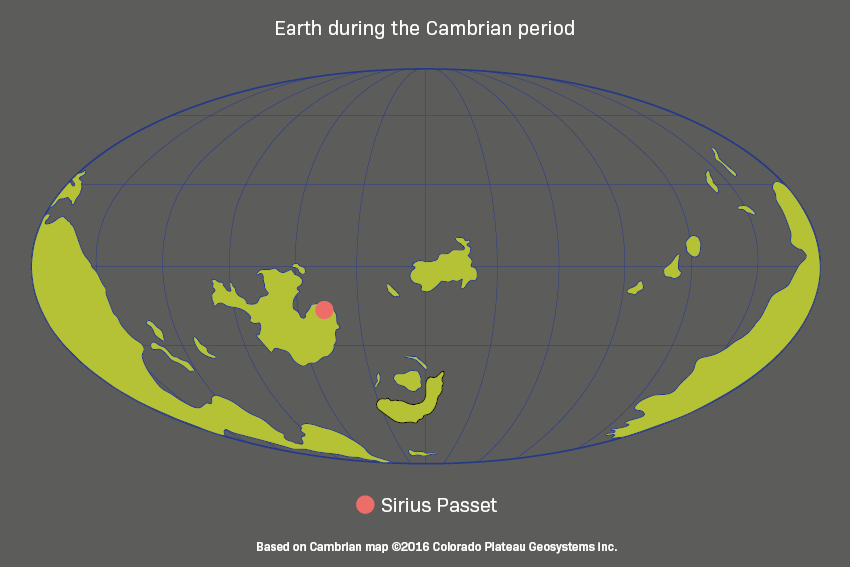
Sirius Passet fossil site, Greenland

Sirius Passet takes its name from the elite Danish military unit, the Sirius Dog Sled Patrol, that monitors Greenland’s high Arctic wilderness.
Sirius Passet takes its name from the elite Danish military unit, the Sirius Dog Sled Patrol, that monitors Greenland’s high Arctic wilderness.
The northernmost part of Greenland, high inside the Arctic Circle, is a remote and challenging place to carry out fieldwork. But it is here, in a location called Sirius Passet, that scientists have found one of the oldest-known examples of soft-bodied fossils from the Cambrian explosion.

Halkieria evangelista is a mollusc with a long slug-like body protected from predators by hundreds of overlapping plates called sclerites, and a large plate at either end. The creature may have had a muscular foot and mouthparts, which allowed it to move slowly over the sediment, grazing on bacterial films.

Pauloterminus spinodorsalis was an arthropod with a shell divided into right and left halves, covering the body and legs. It probably swam through the water and captured soft prey items using pairs of appendages near the head. Two other arthropod fossils, including Campanamuta, appear on the slab.
Sirius Passet is around 518 million years old. The species here once lived in deep water far from shore, on a gentle slope, some burrowing into the sediment and others grazing on the ocean floor. Analysis shows they lived in low-oxygen conditions and were preserved when they became engulfed by sediment flows.
Chengjiang fossil site, Yunnan province, China

With its exceptionally preserved record of a complex marine community, Chengjiang is a UNESCO World Heritage site.
With its exceptionally preserved record of a complex marine community, Chengjiang is a UNESCO World Heritage site.
A hilly region of Yunnan province in southwest China is the location of the colourful Chengjiang fossil site. Like Sirius Passet, it is about 518 million years old, from the time when this region was underwater and swarmed with life.
Now, the site gives the most complete record of an early Cambrian marine community, with around 200 identified species including many soft-bodied creatures unknown elsewhere. Under the weathering of a tropical climate, iron oxides in the fossils have turned them rust-red, while the surrounding stone is weathered yellow.

Cricocosmia jinningensis had a distinctive snout called a pharynx, covered in spiny tooth-like structures. The pharynx could be retracted into the spiny proboscis or turned outwards to capture prey. Cricocosmia was a predator that buried itself in the sea-floor sediment and fed on slow-moving animals that it ambushed from its burrow.

Galeactena hemispherica . Modern ctenophores – called comb jellies – all have soft bodies. Galeactena and its relatives, however, were lightly armoured, with hardened spokes running down the sides of the body, and had eight sets of comb rows, which were covered by hair-like cilia in life. This enabled the creature to swim through the water, well above the sea floor. Galeactena lacked the feeding tentacles of living ctenophores and may instead have fed by engulfing its prey.

Burgess Shale fossil site, British Columbia, Canada

Fossils in the Burgess Shale are two-dimensional and preserve soft tissues, like those in the other Cambrian sites. They reveal a huge variety of animal body plans, some strange and others very familiar.
Fossils in the Burgess Shale are two-dimensional and preserve soft tissues, like those in the other Cambrian sites. They reveal a huge variety of animal body plans, some strange and others very familiar.
The Burgess Shale has played a huge role in shaping our understanding of how animal life evolved. High up in the snowy Canadian Rockies, the layered rocks revealed for the first time evidence of the thrilling diversity of species during the Cambrian explosion.
Now over 2000 metres above sea level, the site contains exceptional fossils of marine animals that lived 508 million years ago, many of which were first found by Charles Walcott in 1909.

Marella splendens had a headshield with backwards-facing spines, a pair of long thin antennae, a segmented body and many pairs of legs. It probably swam just above the sea floor and sifted through the mud searching for food.

Ogygopsis klotzi is very abundant in the Burgess Shale, and was a large trilobite that would have scoured the sediment for food.


Halkieria evangelista is a mollusc with a long slug-like body protected from predators by hundreds of overlapping plates called sclerites, and a large plate at either end. The creature may have had a muscular foot and mouthparts, which allowed it to move slowly over the sediment, grazing on bacterial films.
Halkieria evangelista is a mollusc with a long slug-like body protected from predators by hundreds of overlapping plates called sclerites, and a large plate at either end. The creature may have had a muscular foot and mouthparts, which allowed it to move slowly over the sediment, grazing on bacterial films.

Pauloterminus spinodorsalis was an arthropod with a shell divided into right and left halves, covering the body and legs. It probably swam through the water and captured soft prey items using pairs of appendages near the head. Two other arthropod fossils, including Campanamuta, appear on the slab.
Pauloterminus spinodorsalis was an arthropod with a shell divided into right and left halves, covering the body and legs. It probably swam through the water and captured soft prey items using pairs of appendages near the head. Two other arthropod fossils, including Campanamuta, appear on the slab.

Cricocosmia jinningensis had a distinctive snout called a pharynx, covered in spiny tooth-like structures. The pharynx could be retracted into the spiny proboscis or turned outwards to capture prey. Cricocosmia was a predator that buried itself in the sea-floor sediment and fed on slow-moving animals that it ambushed from its burrow.
Cricocosmia jinningensis had a distinctive snout called a pharynx, covered in spiny tooth-like structures. The pharynx could be retracted into the spiny proboscis or turned outwards to capture prey. Cricocosmia was a predator that buried itself in the sea-floor sediment and fed on slow-moving animals that it ambushed from its burrow.

Galeactena hemispherica . Modern ctenophores – called comb jellies – all have soft bodies. Galeactena and its relatives, however, were lightly armoured, with hardened spokes running down the sides of the body, and had eight sets of comb rows, which were covered by hair-like cilia in life. This enabled the creature to swim through the water, well above the sea floor. Galeactena lacked the feeding tentacles of living ctenophores and may instead have fed by engulfing its prey.
Galeactena hemispherica . Modern ctenophores – called comb jellies – all have soft bodies. Galeactena and its relatives, however, were lightly armoured, with hardened spokes running down the sides of the body, and had eight sets of comb rows, which were covered by hair-like cilia in life. This enabled the creature to swim through the water, well above the sea floor. Galeactena lacked the feeding tentacles of living ctenophores and may instead have fed by engulfing its prey.

Marella splendens had a headshield with backwards-facing spines, a pair of long thin antennae, a segmented body and many pairs of legs. It probably swam just above the sea floor and sifted through the mud searching for food.
Marella splendens had a headshield with backwards-facing spines, a pair of long thin antennae, a segmented body and many pairs of legs. It probably swam just above the sea floor and sifted through the mud searching for food.

Ogygopsis klotzi is very abundant in the Burgess Shale, and was a large trilobite that would have scoured the sediment for food.
Ogygopsis klotzi is very abundant in the Burgess Shale, and was a large trilobite that would have scoured the sediment for food.
Animated reconstruction of the Cambrian sea floor, 518 million years ago, based on fossils from Chengjiang, Yunnan Province, China. Video by Mighty Fossils.
Animated reconstruction of the Cambrian sea floor, 518 million years ago, based on fossils from Chengjiang, Yunnan Province, China. Video by Mighty Fossils.
Why did it all happen when it did?
The Cambrian explosion created a complex watery world of animal species that would later move onto the land and into the air. Change took place on a global scale as well as at the most fundamental biological level.
But what set things in motion? Scientists are still weighing up the factors that led to the origin of animals and their wide variety of body plans. Was it due to a genetic revolution that began within the creatures themselves? Was it down to environmental change that created new habitats and allowed higher levels of biodiversity? Or were both essential?
We don’t yet know but while the incredible fossils from sites such as Sirius Passet, Chenjiang, and Burgess Shale help us reach back into deep time and see the first animals with more clarity than ever before.
Paul Smith, director of Oxford University Museum of Natural History, outlines the conditions that gave rise to the Cambrian explosion
Paul Smith, director of Oxford University Museum of Natural History, outlines the conditions that gave rise to the Cambrian explosion
Evolution of modern animal life
Imagine the world of the Cambrian, without mammals, birds or insects. There are no trees or flowering plants. Nothing moves over the planet’s dry land. All the action is underwater.
The fossils displayed here represent an extraordinary shift in the nature of life on Earth. Previously, bacteria and other single-celled organisms had dominated the oceans. During the Ediacaran and Cambrian periods, the first modern oceanic ecosystems developed, with recognisable food chains, predators and prey. We find evidence of all the major body plans here, allowing scientists to trace the origin and evolutionary journey of most of the animal groups we have today.
Whether a modern creature has four legs, or forty, or none… whether it has claws, teeth, wings or feelers… whether it is shelly or scaly… its fundamental structure was set half a billion years ago, as is illustrated in the following images.
In this special debate for our First Animals exhibition programme scientists discuss the extraordinary evolutionary event that sparked the diversification of animal life.
In this special debate for our First Animals exhibition programme scientists discuss the extraordinary evolutionary event that sparked the diversification of animal life.
Interpretation Oxford University Museum of Natural History Public Engagement Team
Research Oxford University Museum of Natural History Research Team
Text Rebecca Mileham
Digital reconstructions and artwork Mighty Fossils
Special thanks for exhibition loans
to:
Yunnan Key Laboratory for Palaeobiology and MEC
International Joint Laboratory for Palaeobiology and Palaeoenvironment, Yunnan Univeristy, Kunming, China
Royal Ontario Museum, Toronto, Canada
University of Bristol, UK
Special thanks to our funders:
EPA Cephalosporin Fund
Arts Council England
Research England